Improving Diversity and Inclusion in Clinical Trials
Ebooks & White Papers
• March 15, 2022
INTRODUCTION
With health equity at the forefront of the conversation today, many clinical trial sponsors are seeking ways to create a more diverse and inclusive research environment.
At BBK Worldwide, we believe that clinical trials for investigational treatments should reflect the populations that could benefit from them, ensuring better health interventions for those in greatest need.

To help increase access to care and support for a diverse and inclusive research environment, we are offering strategic guidance that sponsors can leverage. As a bonus feature, we are sharing excerpts from a recent Q&A with Michele Russell-Einhorn, chief compliance officer and institutional official at Advarra, that provide an IRB’s perspective on improving diversity and inclusion in clinical trials.
Driving the Conversation

The renewed focus on improving representation in clinical trials has been driven, in part, by the disparate impact of COVID-19 on racial, ethnic and underserved communities. Furthermore, with the issuance of its latest guidance, Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry, the FDA has signaled the need to do more to recruit and retain patients from traditionally underrepresented trial populations. With this FDA research considered alongside statistics that show a dramatic gap in representation, it is clear that the industry must take measures now to create a more equitable recruitment environment.
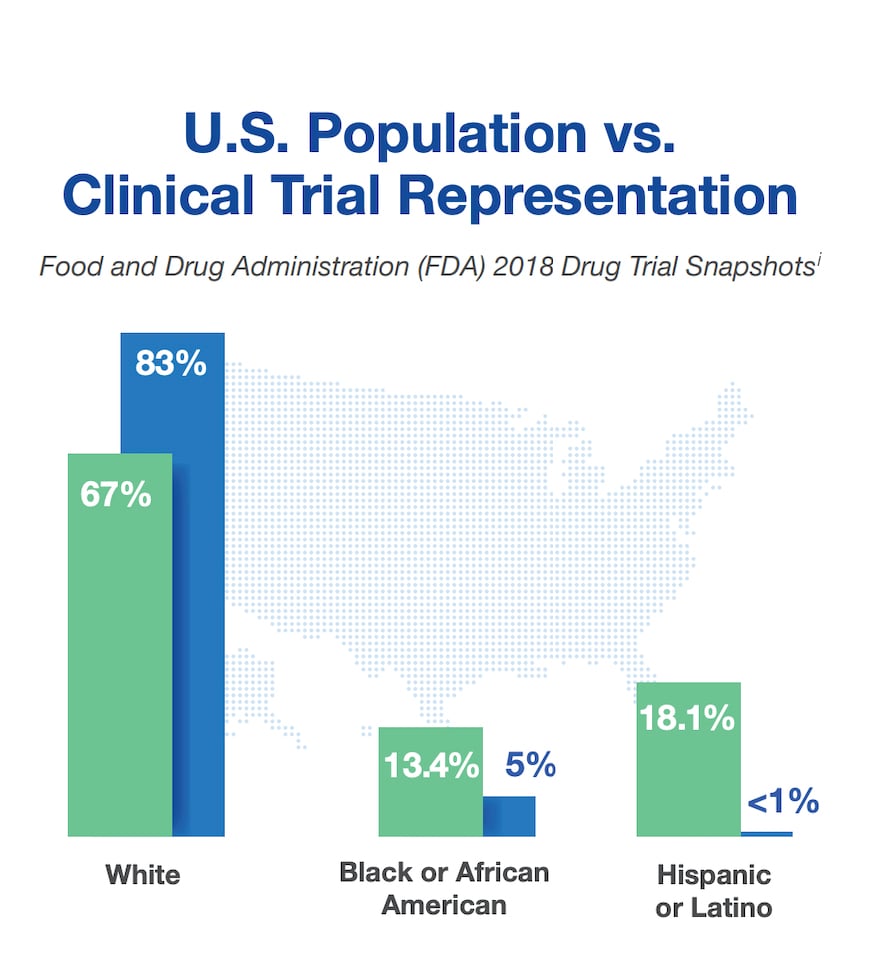
Representation at a Glance
The U.S. Food and Drug Administration (FDA) found significant imbalance in representation of minorities in clinical research as compared to the U.S. population.
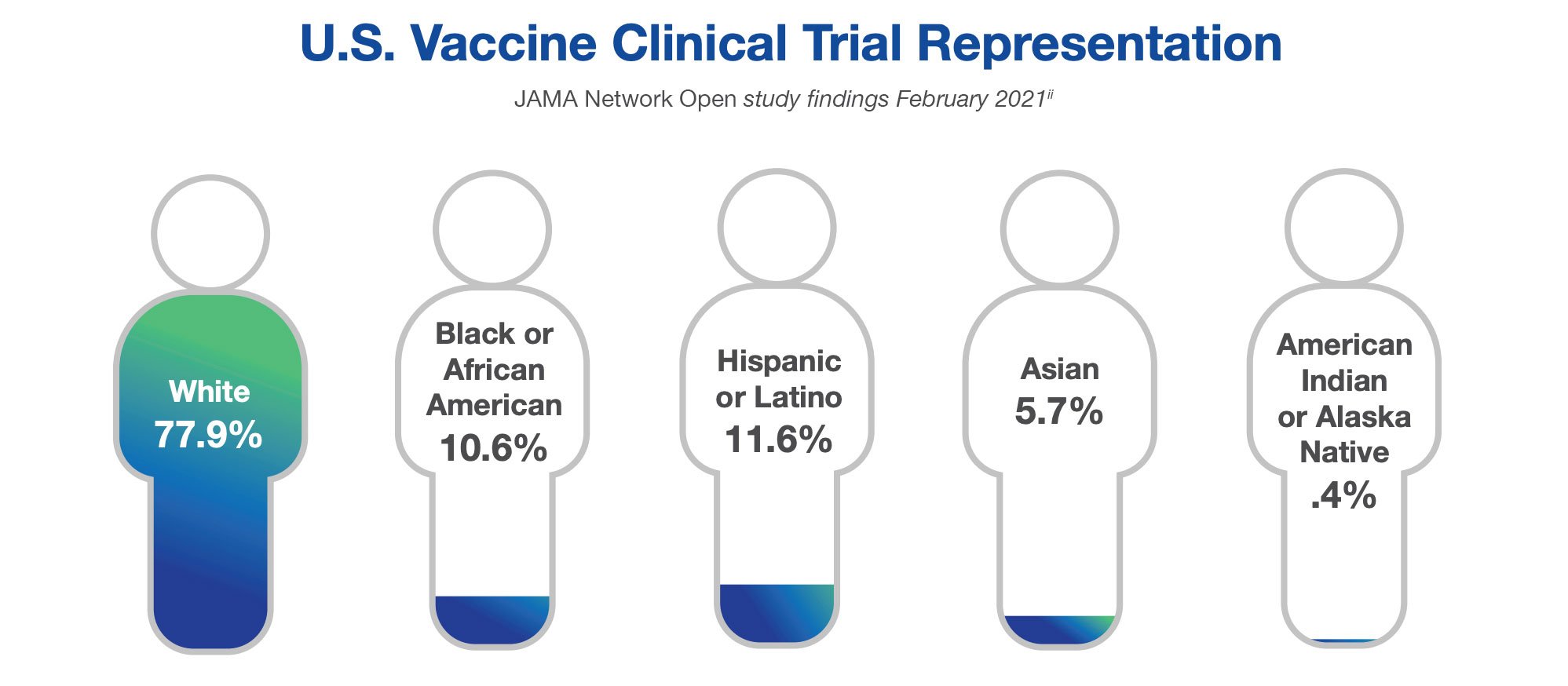
Similar findings were reported in interventional vaccine trials, by the JAMA Network Open study. The study captured data from July 2011 to June 2020 and was published in February 2021.
i. Yates I, Byrne J, Donahue S, McCarty L, Mathews A. Representation in clinical trials: a review on reaching underrepresented populations in research. ACRP. Clin Research. 2020;34(7). https://acrpnet.org/2020/08/10/representation-in-clinical-trials-a-review-on-reaching-underrepresented-populations-in-research
ii. Flores LE, Frontera WR, Andrasik MP, et al. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Netw Open. 2021;4(2). https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2776562
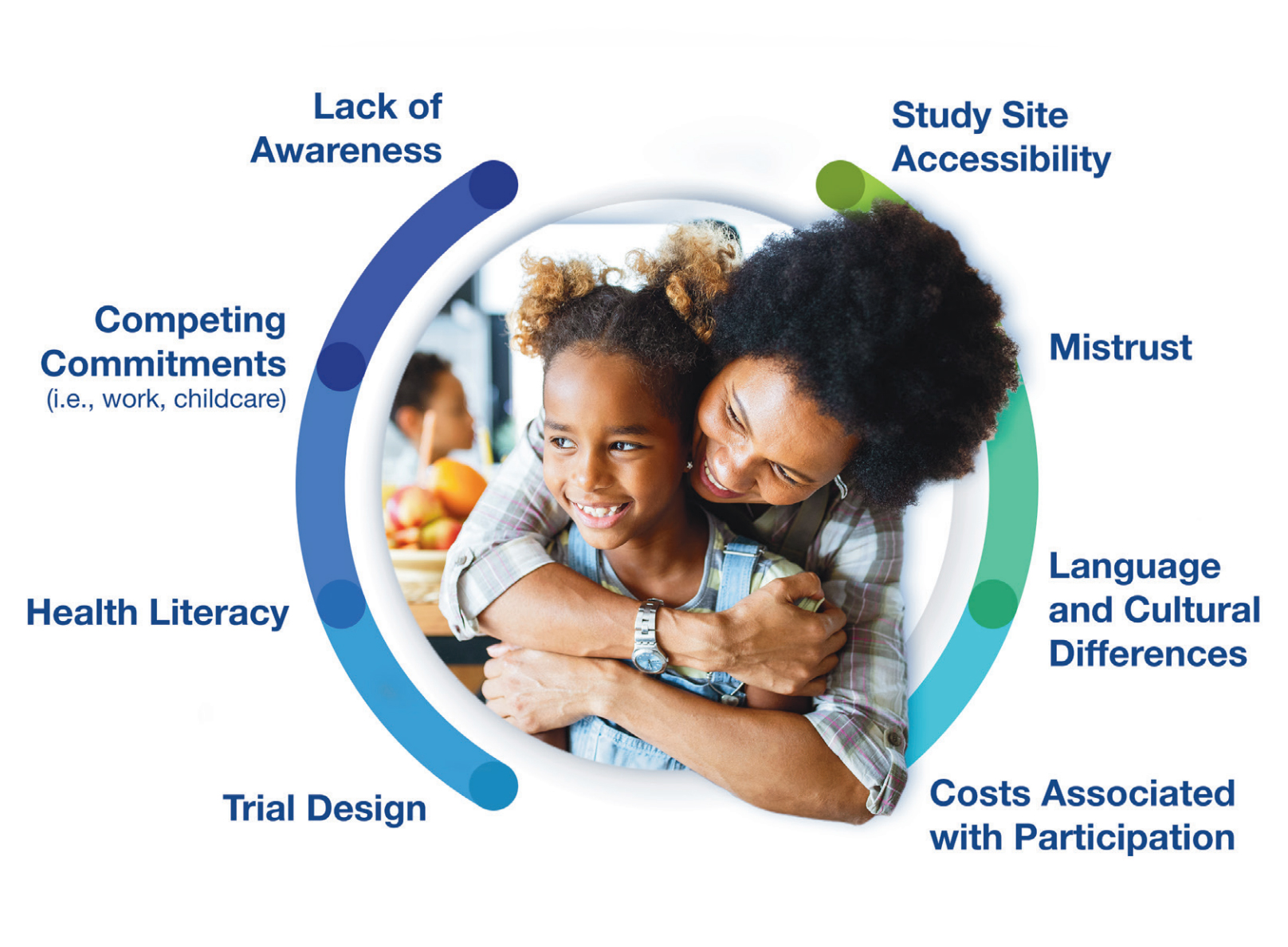
Common Barriers to Participation
Our guidance reinforces the importance of understanding the audience — what they care about, how they feel and what motivates them.
To be effective, you need to determine your target audience’s key habits and trusted influencers in the healthcare decision-making process and identify any barriers that may need to be overcome. This extends to recognizing if the trial design contains a burdensome visit schedule that might be problematic for a patient juggling work and childcare duties; or if the study site is situated in a location that may not be easily accessible by public transportation; or even if negative perceptions of clinical research are influencing consideration.
Leverage Strategies to Help You Improve Clinical Trial Diversity and Inclusion
Ease Visit Burden
Consider replacing certain in-person visits with telehealth. For routine labs, consider engaging a local lab. Utilize digital health technologies to capture routine health measures.
Broaden Eligibility Criteria
Ensure that the eligibility criteria serves the goal of having a representative sample of the population for whom the drug is intended.
Remove Financial Obstacles
Make sure you are reimbursing costs associated with participation, such as transportation, meals, childcare and lost wages.
Improve Convenience
Consider if a virtual or hybrid study model is appropriate in order to limit the number of in-person visits. Conduct certain visits at satellite locations to enhance convenience.
Increase Awareness to Technology
Provide participants with tablets and iPhones, as well as hotspots for internet access, to facilitate participation and compliance.
Conduct the Study Locally
Consider opening a site in the local community to increase access and make participation more approachable. Consider extending site office hours to evenings and weekends.
Build Trust
Overcome mistrust and skepticism with transparent dialogue and education about the clinical research process. Collaborate with trusted community leaders.
Culturally Adapt Materials
Minimize language barriers by translating materials and making them culturally relevant for your audience.
Know Your Audience
Conduct market research to gain insight into your audience — including work habits, media preferences and hobbies — and leverage findings to inform outreach and advertising tactics.
Engage Locally
Consider sending a study educator to community health centers, town meetings and church gatherings. Implement grassroots marketing efforts to engage with the community.
Q&A WITH ADVARRA
Diversity and Inclusion in Clinical Research: The IRB’s Perspective
BBK Worldwide Creative Director for Patient Experience and Engagement Jessica Kim recently spoke with Michele Russell-Einhorn, chief compliance officer and institutional official at Advarra, on the topic of underserved populations.
Below are excerpts from the conversation.
Jessica: Are institutional review boards, or IRBs, now evaluating protocols for diversity and inclusion? Do you feel it’s often too late by the time the protocol comes to the institutional review board?
Michele: The institutional review board has the very significant and important role of protecting people in research. One of the specific 111 criteria for IRB review is to make sure subject selection is equitable. If the research protocol is being conducted in an urban area and the exclusion criteria say you have to be able to speak English, the IRB — and this is prepandemic — should always raise a question: Why would you limit the protocol to only individuals that speak English when 50% of the urban population where this is being conducted are Spanish-speaking individuals? I would like to think that IRBs have always felt empowered to ask that question.
The problem is that having multiple languages can be a resource issue for investigators. Do they have the resources to have informed consent forms translated or to use what we commonly call short-form consent?
It doesn’t stop there because you have to have interpreter services. Just because you conduct the consent process in somebody’s language doesn’t mean you get to forget about it afterward. You have to make sure that you have resources following them.
One of the interesting things from the pandemic is that a lot of the research was clinic- and hospital-based research. Then the pandemic hit, and all of a sudden we have a move to telemedicine and home health visits. What I think we have seen as a result is something that supports the inclusion of a more diverse population.
If you looked at the procedures outlined in the protocol and it said you have to come to the clinic or to the hospital or to the office for study treatment 12 times, that’s a huge amount of time for an individual to take to travel and to leave work. But if they can stay home, or if they do not have to travel so far, you have the likelihood of engaging a more diverse population in the research. That said, I think it is reasonable for an IRB to ask at the time of review, “Do you really need 12 procedures to be done at your clinic, and is there a way for you to incorporate telemedicine or visits by home health aides?”
It would be hard-pressed for an IRB to actually require a procedural change that is integral to the protocol design — it could affect the integrity of the research and how the research is being envisioned. It doesn’t mean it can’t be suggested, and I do think that is an appropriate route for an IRB to go.
Jessica: What is the IRB’s view on things such as hotspots and iPhones being provided to underserved populations in order to be involved in clinical research, especially when they may not have access to internet or a smartphone?
Michele: The incorporation of digital technology in research is something we have seen expand over the last couple years. There is no prohibition on providing iPads, iPhones, Apple watches, anything you want in the context of research if it’s related to the research and used to facilitate communication with a participant.
But what you really have to think about is does the participant get to own it? Does the participant have to return it? What are you going to do if an iPhone is not returned? If there is no financial cost to the participant and nothing else arises, then it shouldn’t be a problem at all. But if the sponsor is going to contemplate some kind of consequence, you have to be very careful.
If you give participants an iPad and expect them to give it back, and they don’t, if you then withhold other compensation, an IRB might not look kindly on that quid pro quo. In summary, there is no prohibition on providing participants with those devices.
Jessica: The FDA issued renewed guidance last November for recruiting and retaining patients for traditionally underserved populations. Do IRBs expect study sponsors to adhere to this guidance?
Michele: The FDA guidance is just that — guidance. It’s not something that is required by regulations. I think when you are engaged in the research enterprise, you are familiar with the guidance documents from the FDA. You do your best to incorporate that guidance as best practice in your research.
On the recruitment front, that guidance is very helpful and provides some very good suggestions to sponsors for the design of research and other aspects related to recruitment. It could well play into how an IRB might come up with a suggestion or a recommendation that a sponsor could consider in the future if they are not able to implement something for the research under review.
Jessica: What are some early considerations that study sponsors can start to think about while designing their clinical trials to be more diverse and inclusive?
Michele: It’s important to think about your catchment area — where are you going to do your recruiting, where are your clinics and health centers? Are they located in areas that will pull a diverse population? And also look at your procedural requirements. Will the procedures dissuade people from wanting to participate in the research because of travel and time commitments? Are there ways to substitute for in-office visits, or are there other ways to participate in the research? So I think those are some of the things to consider at early protocol design.
LISTEN TO THE PATIENT VOICE
How Do You Incorporate Choice?
Start by asking questions. Learn what matters to patients. Know their attitudes. Understand their needs. For instance, do they have access to high-speed internet? Do they have their own tech device, or do they need an iPhone or tablet in order to participate in a clinical trial? How tech-savvy are they? Do they have a car, or do they rely on public transportation? Are up-front out-of-pocket expenses a concern?
Then leverage a hybrid approach to help fill in the gaps. For instance, for patients who indicate they are not tech-savvy, consider more avenues for education and support — it doesn’t mean you have to exclude them from participation. A hybrid approach provides the agility that maximizes engagement among all audiences.

Survey Methodology
The Study Voices 2021 survey findings include responses from 2,078 healthcare consumers.
The survey was conducted in the United States between August 18 and August 29, 2021.
Subscribe to stay connected
BBK Worldwide, LLC
117 Kendrick St., Suite 600
Needham, MA 02494 United States
Terms & Conditions Privacy Policy
© 2025 BBK Worldwide. BBK Worldwide is a Publicis Health company.
Terms & Conditions Privacy Policy
© 2025 BBK Worldwide. BBK Worldwide is a Publicis Health company.

