Blog
• January 31, 2023

 Rob Laurens
Rob Laurens
Chief Patient and Provider Officer
The EU Clinical Trials Regulation (CTR) replaces the Clinical Trials Directive 2001 / 20 / EC and looks to ensure a unified set of rules for the conduct of clinical trials throughout the European Union. From the outset, policymakers had the aim of making the EU more attractive to clinical trials by simplifying submissions, authorizations and conduct among EU member states, thereby also offering greater transparency into trial data.
A key piece of this is the procedure of submission of all trial documentation through a single portal called the Clinical Trial Information System (CTIS), found at https://euclinicaltrials.eu/home.
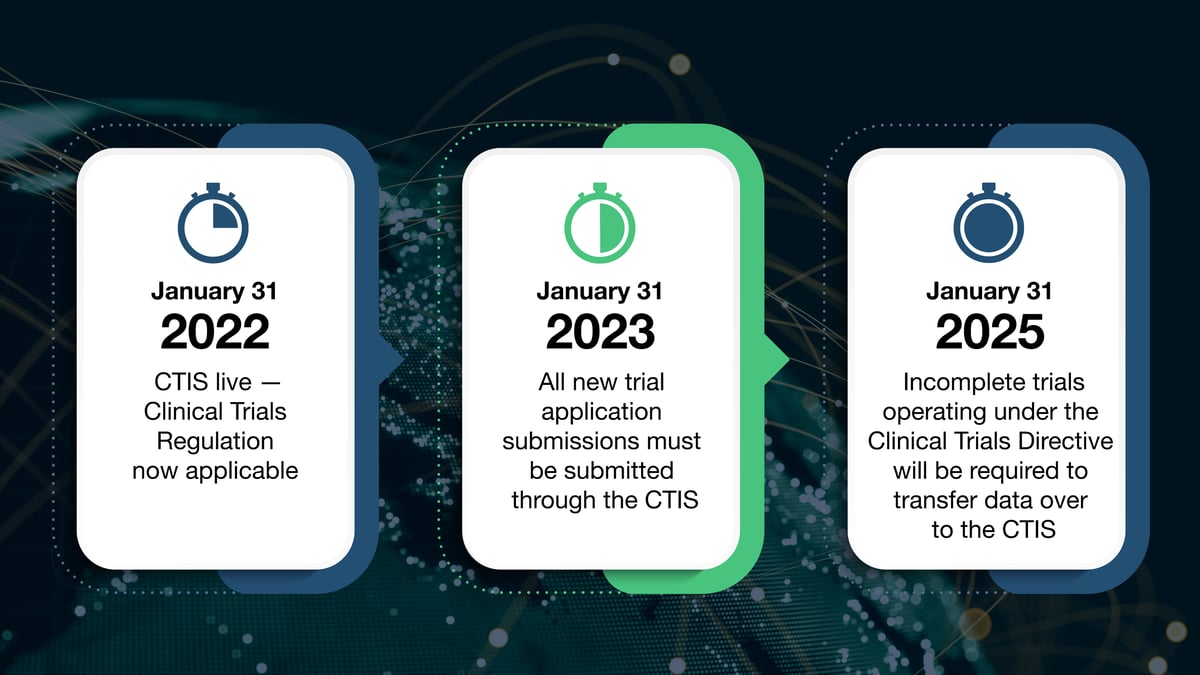
Although the CTR was authored back in mid-2014, it was delayed due to technical difficulties with the development of the CTIS. After an independent audit in 2020, the commission published notice of full functionality in 2021. Subsequently, the CTIS went live, and the date of applicability of the Clinical Trials Regulation was fixed on January 31, 2022.
This date has initiated a three-year transition period, during which:
There is a one-year grace period where clinical trial applications can be set up through the CTIS, but it is not a legal requirement.
Beginning January 31, 2023, all new trial application submissions must be submitted through the CTIS.
Any studies already underway will be grandfathered under the outgoing Clinical Trials Directive. All grandfathered studies will have until January 2025 to be completed, or they too will be required to transfer their data over to the CTIS.
From a positive perspective, the CTIS single submission requirement eliminates the need for multiple submissions in all participating EU countries and, perhaps more importantly, the need to understand and follow the complex and changing procedures and regulations for each country. In theory, then, submitting all trial documentation once through the CTIS should also:
minimize errors and ensure a higher overall quality of data
dramatically shorten timelines for all participating countries to first-patient-in (FPI)
create a more predictable timetable for the commencement of enrollment
In other words, the CTR and CTIS will have the positive effect of simplifying the process of conducting clinical trials in the EU — but not without some potentially painful adjustments for sponsors.
In the pre-regulation environment, clinical trial managers tend to focus on FPI in the country most likely to receive the quickest approvals. All other participating EU countries are activated according to their own timelines and operate independently with respect to their judgment around the need for patient recruitment planning and activities. All this adds up to a chaotic jumble of country and site activations with little to no coordination between country and site managers; a suboptimal, poorly planned number of active recruiting site months among selected sites; and a confused, inefficient recruitment strategy when sites are finally activated.
Now, with the CTIS and single submission in place, the focus fundamentally has to shift away from a sequence of independent country submissions toward a coordinated planning approach that hastens FPI in the country least likely to receive the quickest approvals. The rate-limiting factor of getting everything in place for that country now becomes the race against the clock for study submission.
In addition, planning and execution for patient recruitment and retention also jump to the fore. Since all patient- and physician-facing recruitment strategies and translated materials and tactics must be submitted at the time of single submission, the timing to consider and create them begins at the point of country selection. Depending on the number of countries and languages required, this type of content may take up to 16 weeks to fully prepare for submission.
In many instances, recruitment and retention strategies and tactics are too far down the list of priorities and become last-minute additions. But this cannot persist in the new environment, and a paradigm shift needs to occur to integrate these unified approaches into clinical trial planning as early as possible.
The timing of the mandatory requirement to use the CTIS on January 31, 2023, offers a sobering reminder that the time to begin planning for recruitment and retention in studies intended to begin after that date is right now.
BBK Worldwide is uniquely positioned to be your partner in this brave new world! Connect with us to learn more about how Regulation (EU) No 536 / 2014 and the CTIS requirements will impact your clinical trial recruitment operations.
A useful link to the CTIS Sponsor Handbook can be found here: https://www.ema.europa.eu/en/documents/other/clinical-trial-information-system-ctis-sponsor-handbook_.pdf

Podcasts & Videos
April 16, 2019

Blog
May 18, 2020
Podcasts & Videos
March 29, 2021
BBK Worldwide, LLC
117 Kendrick St., Suite 600
Needham, MA 02494 United States
Terms & Conditions Privacy Policy
© 2025 BBK Worldwide. BBK Worldwide is a Publicis Health company.
Terms & Conditions Privacy Policy
© 2025 BBK Worldwide. BBK Worldwide is a Publicis Health company.