Tenosynovial Giant Cell Tumor also known as pigmented villonodular synovitis (PVNS)
PHASE 3
Overview
A rare and difficult-to-treat disease with patients who had suffered from multiple misdiagnoses, failed surgeries, recurring symptoms and lingering life-altering discomfort: How do you recruit a patient population with sustained frustration with the medical community? BBK Worldwide was up to the task.
We were engaged to help recruit this Phase 3 study examining an oral, investigational drug for patients with tenosynovial giant cell tumor (TGCT), also known as pigmented villonodular synovitis (PVNS) — a condition that causes persistent and intense joint pain.
The key proved to be a targeted recruitment campaign that conveyed empathy for the difficult journey these patients had endured, while inspiring them to feel empowered to take a positive step “forward” toward an improved quality of life.
We helped patients reimagine a future in which progress could be possible.
Challenges
-
Notably low disease prevalence
-
Patients frustrated from repeated misdiagnoses,
lack of surgical options, long-term pain and feeling demoralized by ultimately ineffective clinical interactions -
Burdensome study duration (61 weeks) and patient commitment (up to 12 cycles of patient visits)
-
The existence of an FDA-approved drug that provided
an alternative to U.S. clinical trial participation -
Paucity of sites and a necessary nationwide advertising campaign requiring physically impaired patients to travel long distances
Solutions
Recruitment success depended on speaking to the largest possible audience. This meant we needed to understand the complete spectrum of patients’ mindsets leading to the moment in which they sought (or were open to) alternative treatment options and clinical trial consideration.
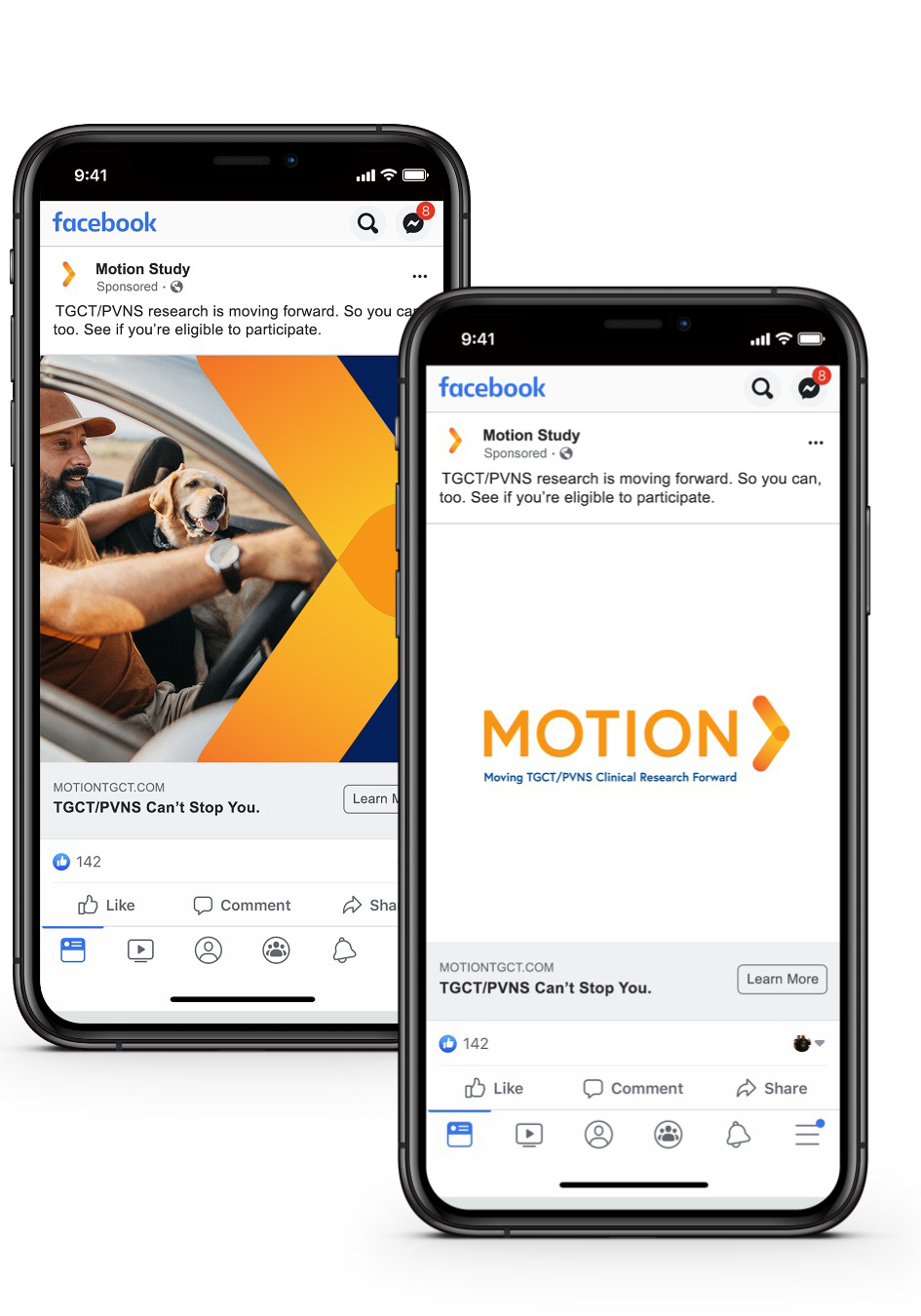
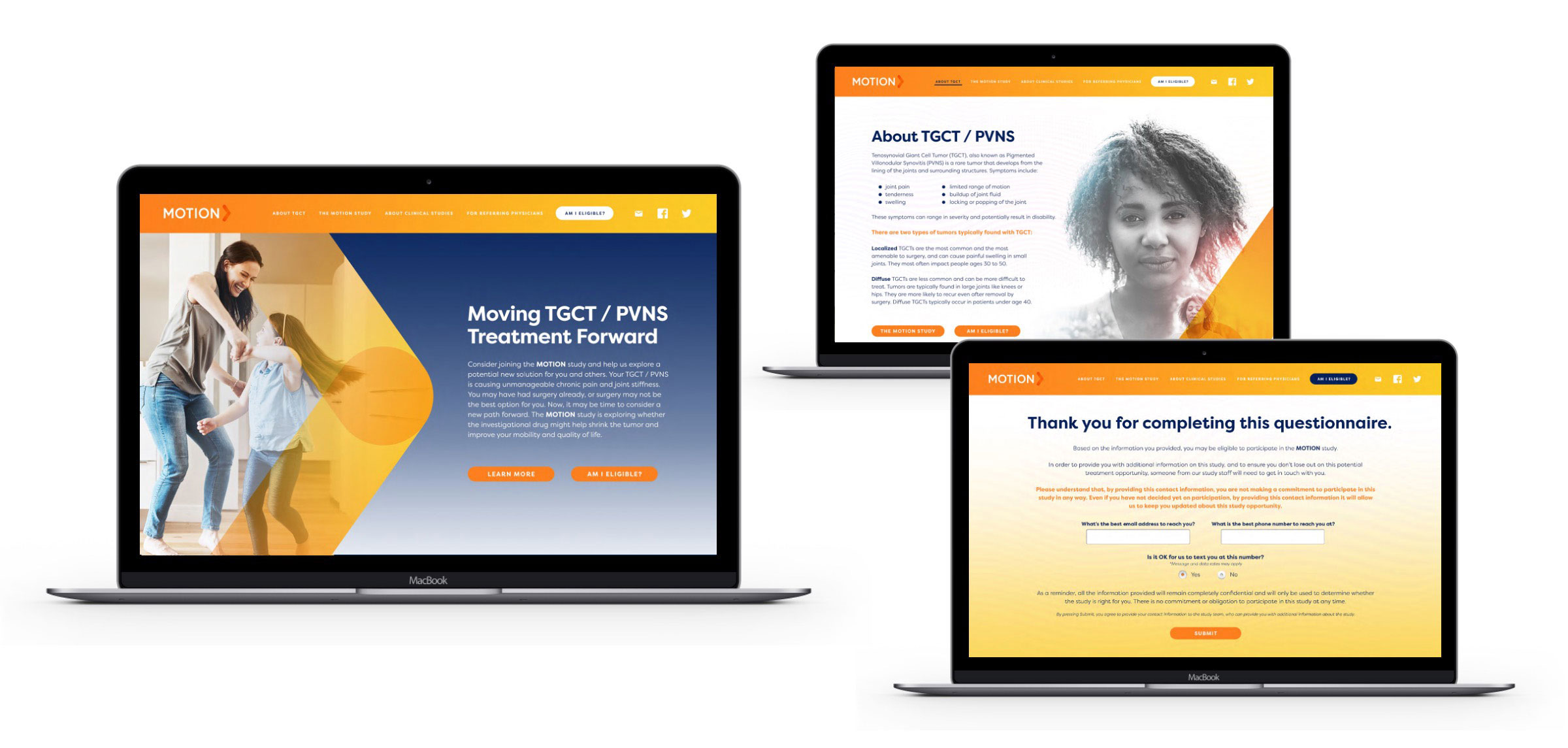
Our national campaign acknowledged TGCT patients’ struggle and sympathized with their frustration and distrust while our study materials honored their perseverance in considering a new approach.
The creation of customized materials designed specifically for patients with these rare conditions was essential to convey the unique availability of an opportunity for people just like them — and spoke to their feelings of being overlooked by the medical community for years. Referring physicians used these tailored materials to support their one-on-one discussions with potential participants.

Impact
BBK designed and implemented a targeted, relevant solution catering to a patient who had felt disserved by the medical community.
BBK deployed outreach with supplemental referral management support to sites to ensure swift follow-up to capture potential participants — recognizing that a study this difficult to recruit couldn’t afford to lose a single referral.
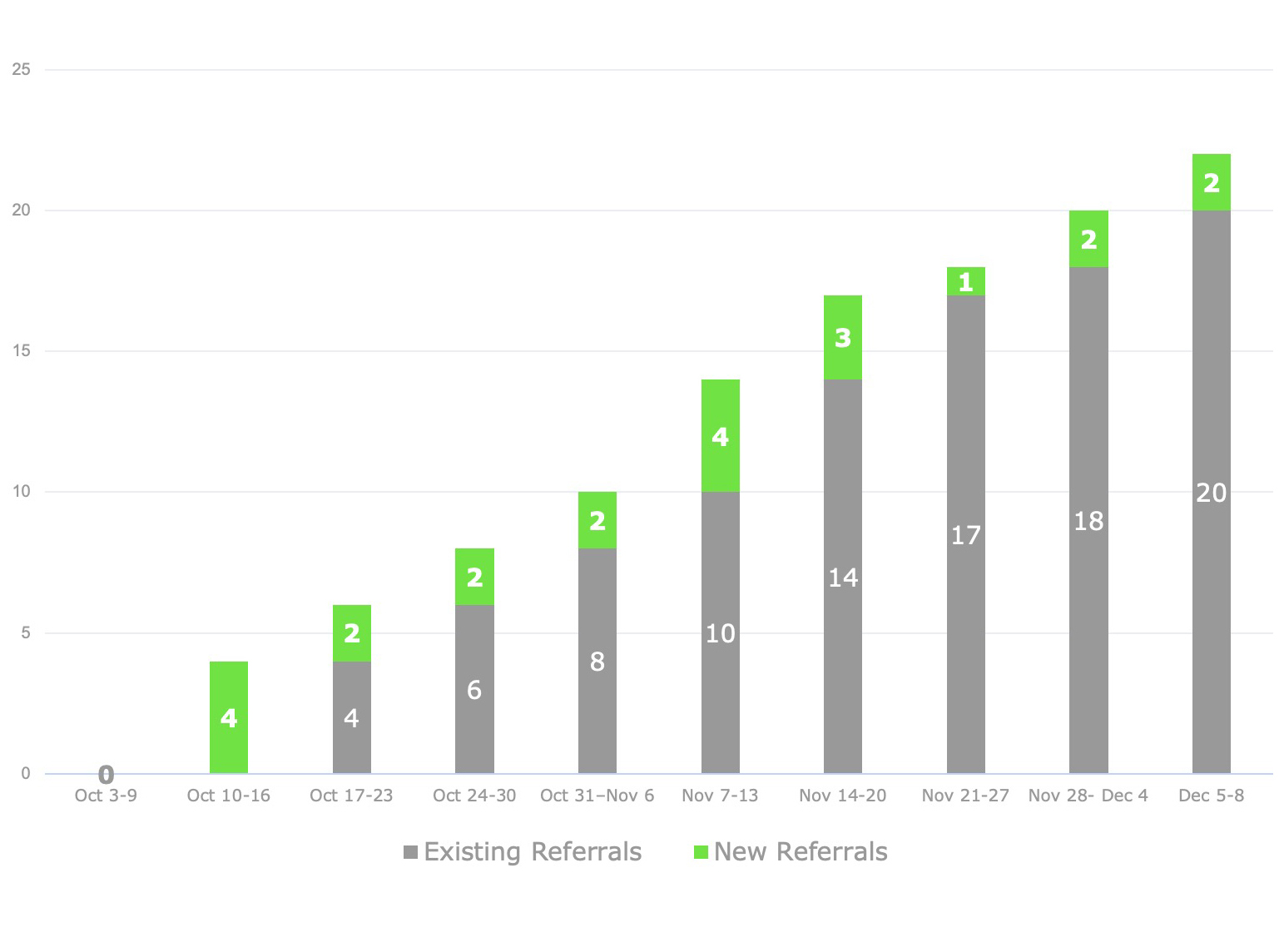
Recruitment for eligible patients proved generative and fruitful, despite the condition rarity, the required travel, and patients’ long-term sense of disempowerment. In the end BBK generated 25 qualified referrals — about twice the projected target.
After years of patients feeling abandoned, we succeeded by promoting the existence of a study designed specifically for them and their unique experience.
-
 25
QUALIFIED REFERRALS
25
QUALIFIED REFERRALS
-
 3.3
MILLION IMPRESSIONS
3.3
MILLION IMPRESSIONS
-
 6
SITES
6
SITES
-
 2.5
MONTHS OF SUPPORT
2.5
MONTHS OF SUPPORT
Referral Numbers by Week
“Our team is quite excited by the number of referrals so far. … Thank you to the BBK team for your tremendous efforts in getting us to this point.”
— Sponsor Study Lead
Download the PDF version of this case study.
Subscribe to stay connected
Related Resources

Familial Amyloid Polyneuropathy — Neurology and Gene Therapy — Phase 3
Case Studies
October 12, 2021

Pediatric Spinal Muscular Atrophy — Neurology and Rare Disease — Phase 2
Case Studies
December 13, 2021

Major Depressive Disorder — Mental Health — Pivotal Phase 3 Study
Case Studies
February 5, 2022
BBK Worldwide, LLC
117 Kendrick St., Suite 600
Needham, MA 02494 United States
Terms & Conditions Privacy Policy
© 2024 BBK Worldwide. BBK Worldwide is a Publicis Health company.
Terms & Conditions Privacy Policy
© 2024 BBK Worldwide. BBK Worldwide is a Publicis Health company.

