Key Takeaways
BBK Worldwide was hired to support the TEMPO trials, a combination of three phase 3 studies and an open-label extension, examining the effects of an investigational drug (tavapadon) in patients with early-onset and late-stage Parkinson’s disease.
The studies were seeking to enroll 1,200 individuals, aged 40 to 80 years.
BBK’s patient recruitment campaign for the TEMPO was selected as a finalist for the Pharmaceutical Executive APEX Awards.

Challenges
Streamlining messaging to support multiple studies.
Creating a sophisticated pre-qualification screener to triage patients to the appropriate study.
Motivating a patient population newly diagnosed with Parkinson’s disease to consider clinical trial participation.
Recognizing the strong possibility that KOLs and historically high-performing sites would be conducting multiple trials recruiting within the same patient pool.
Solutions
BBK’s efforts focused on general Parkinson’s disease awareness to drive interested and potentially eligible patients to sites for further screening and assessment.
Patient-facing materials included a mix of program-level materials (used across all protocols), as well as protocol-specific materials (tailored to include study-specific information).
BBK prioritized the development of a website to inform and prescreen individuals recently diagnosed with Parkinson’s disease.
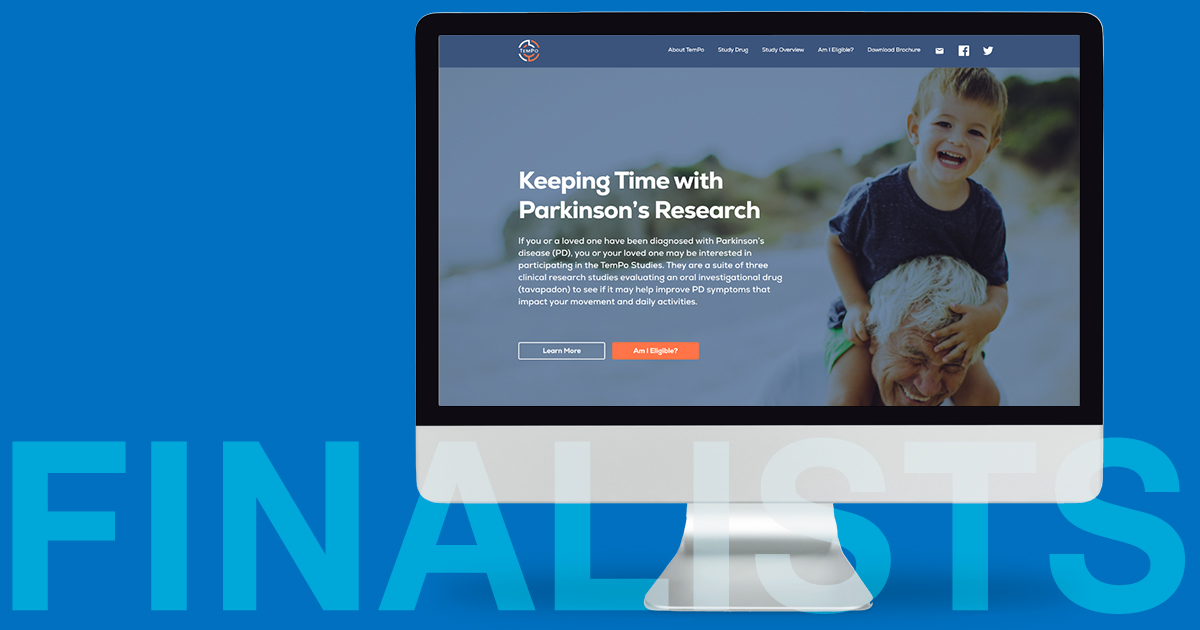
The website had a sympathetic tone with the understanding that patients recently diagnosed were looking to make the most of their time and quality of life in the face of this progressive disease. The campaign tagline — Keeping Time with Parkinson’s Research — reinforced this concept.

Impact
The website and prescreener were live for 14 months in the United States, garnering 1,639 referrals across three protocols — far exceeding the 760 initially projected.
The study was supported by a call center with representative proactively reaching out to website referrals prior to site assignment to verify information. This added step helped ensure sites were able to reach and engage the majority (65-76%) of interested patients.
-
 879
The campaign drove 879 more referrals than initially projected.
879
The campaign drove 879 more referrals than initially projected.
-
 1,639
BBK generated a total of 1,639 qualified referrals for the study, helping the sponsor achieve the enrollment goal of 1,200 patients.
1,639
BBK generated a total of 1,639 qualified referrals for the study, helping the sponsor achieve the enrollment goal of 1,200 patients.
Pharmaceutical Executive APEX Awards
The campaign was selected as a finalist for the Pharmaceutical Executive APEX Awards, the only healthcare marketing awards judged entirely by healthcare professionals (HCPs). BBK was honored to be recognized by those who understand what it takes to build trust with the Parkinson's disease community.
Download the PDF version of this case study today:
Subscribe to stay connected
Related Resources

Familial Amyloid Polyneuropathy — Neurology and Gene Therapy — Phase 3
Case Studies
October 12, 2021

Pediatric Spinal Muscular Atrophy — Neurology and Rare Disease — Phase 2
Case Studies
December 13, 2021

Major Depressive Disorder — Mental Health — Pivotal Phase 3 Study
Case Studies
February 5, 2022
BBK Worldwide, LLC
117 Kendrick St., Suite 600
Needham, MA 02494 United States
Terms & Conditions Privacy Policy
© 2024 BBK Worldwide. BBK Worldwide is a Publicis Health company.
Terms & Conditions Privacy Policy
© 2024 BBK Worldwide. BBK Worldwide is a Publicis Health company.

